|
Cardiovascular Biomechanics and A.I. Laboratory |
|
|
Imperial College London, Department of bioengineering |
|
Advancements
to Heart Function Evaluation |
|||||||
|
Motivation Echocardiography evaluation
of cardiac function is important to diagnose, prognose and time intervention.
Several current echocardiography approaches to evaluating cardiac function
have limitations. We worked to improve several of them. The
Corrected Ejection Fraction (EFc) The Ejection
Fraction (EF) is widely used clinically to evaluate cardiac health, as low EF
can indicate poor outcomes and the need for treatment. However, EF is
affected by geometric changes to the heart due to cardiac remodelling, and in
such instances, is no longer a good indicator of function. For example, EF
tend to be high in hypertrophic hearts, and this prevented it from indicating
a low cardiac function during HFpEF (heart failure preserved ejection
fraction) hearts. We developed a corrected EF, called the EFc, that resolves
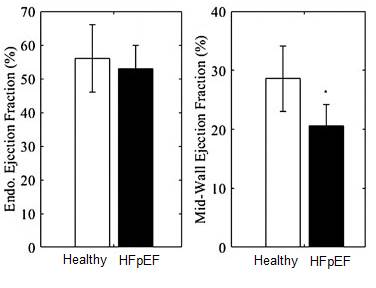
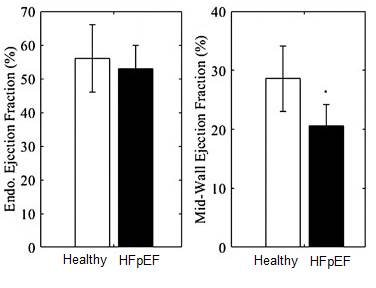
this dependency on cardiac geometry, and showed that it can distinguish
healthy and HFpEF heart. We also showed that EFc has improved ability for
prognosis of re-hospitalization due to heart failure. Our proposed EFc
is equivalent to computing EF at the mid-wall location (between endocardial
and epicardial boundaries), rather than computing it at the endocardial
boundary. It can be calculated from routine echo scan parameters as:
EDV is the LV
end-diastolic volume, and r is the density of myocardium, and LVM is
the left ventricular mass, which can be calculated via the Devereux formulae.
We used a
computational model to study the midwall-EF measure, we find that when the
heart is thickened with no change to contractile strain, EF tend to increase,
and when the heart is dilated with no change to strain, EF tend to decrease.
EFc, on the other hand, is independent of these geometric changes. We also
find that using the mid-wall EF, we can distinguish between HFpEF and Healthy
hearts from an animal model of HFpEF, and from clinical data.
Using a
computational model, we gauged whether geometric changes to the left
ventricle (thickening of walls and dilating the chamber) at no change to the
contractile strain will cause changes to EF or EFc. We find that EF is
geometrically dependent, while EFc is not.
We performed cox proportional
regression modelling to test EF and EFc's relative prognosis value in
predicting re-hospitalization due to heart failure within 3 years, in a
cohort of 2752 patients. We find that in the sub-cohort where EF is in the normal range (>
50%), using EFc rather than EF in the model increased true
positive by 12.2% and decreased false negative by 16.6. ROC
analysis showed 18.6% reduced error in predicting readmissions with EFc
rather than EF. This suggested that EFc had a better accuracy
in predicting re-hospitalization outcomes.
3 years
non-admission ROC curve for patients with ejection fraction ≥ 50 using a leave one out cross validation of various
predictive models, "Baseline" is a cox
proportional hazards regression model where risk of readmissions due to heart
failure within 3 years is predicted from age, gender and blood creatinine
data. "Baseline+EF" is the model where EF is added as predictor,
while "Baseline+EFc" is where EFc is added as a predictor. The area under
the curve (AUC) is given in the legend. p-value = 0.007. References:
- Zheng Y, Chan WX, Charles CJ, Richards AM, Sampath S, Ali AAB, Leo HL and Yap CH. "Effects of Hypertrophic and Dilated Cardiac Geometric Remodeling on Ejection Fraction" Frontiers in Physiology. 2022:1068 3D
Echocardiographic Fetal Heart Strain Measurements We
used our cardiac motion tracking algorithm on fetal echocardiography images,
to compare and understand the differences between 2D versus 3D strain
measurements. 2D images were directly extracted from 3D fetal echo images for
controlled experiments, the same algorithm was used to track both types of
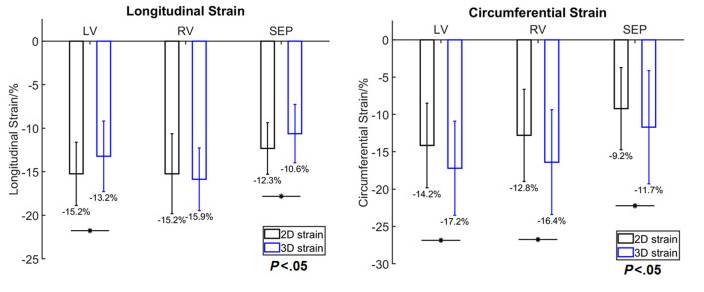
strains. We find that 2D longitudinal strains are underestimated in the LV
compared to 3D strains, while 2D circumferential strains were overestimated
in the LV, RV and septum compared to 3D strains.
We
discovered basic mechanisms that can explain specific biased differences
between 2D and 3D echo strain measurements. One such mechanism was the
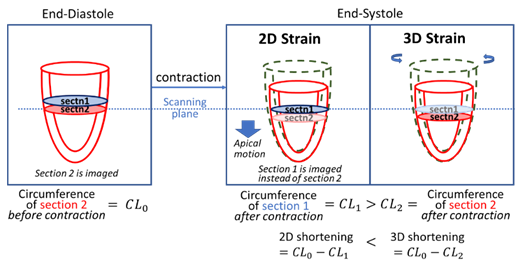
twisting and apical motion of the LV during contraction, as explained below.
Another was that the timing at which the circumferential length and the
longitudinal length was mismatched. 3D measurements needed a single time
frame for zero strain reference, while 2D could have separate time frames for
longitudinal and circumferential strains. Thus there are essential biases in
2D measurements, and 2D strain values should be interpreted with care during
cardiac function evaluation.
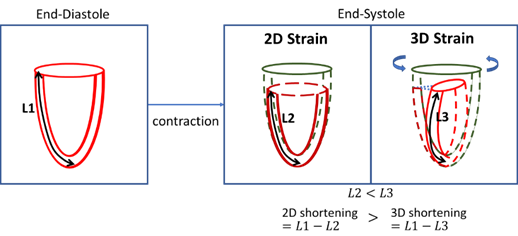
Measurements
of LV longitudinal strain is always higher in 2D than 3D, because of LV
twisting preserves the length of the myocardium during longitudinal
shortening.
Measurements
of LV circumferential strain is always lower in 2D than 3D, due to the motion
of the LV towards the apex during contractions. This apical motion brings
about a wider slice of the LV into the imaging plane to negate
circumferential contractions in 2D scans. |