|
Cardiovascular Biomechanics and A.I. Laboratory |
|
|
Imperial College London, Department of bioengineering |
|
Virtual
Reality Fetal Echo |
||||||||
|
Overall
Methodology Our
overall methodology is to obtain 4D clinical ultrasound images of human fetal
hearts, using the STIC mode. A novel in-house image-registration technique is
applied to accurately extract cardiac wall motions, preserving the cyclic
nature of motions and accurate stroke volume. Finally dynamic mesh
computational fluid dynamics simulations to understand fluid forces and
patterns, and finite element modelling of the myocardial mechanics is
performed to understand myocardial stresses and cardiac function.
|
|||||||||
|
|
Fluid
Dynamics of Normal Fetal Hearts Studies
have been conducted on the normal left ventricle (LV) and right ventricle
(RV) at 22 weeks and 32 weeks old time points. Common flow features were
observation In most hearts hearts, such as a pair of diastolic vortex rings
corresponding to the E- and A-wave, which interacted with each other via
vortex merging or leapfrogging, and which exited the heart before complete
dissipation. The vortex rings were the primary mechanism of elevated wall
shear stress stimuli on the fetal ventricular walls, since they bring faster
moving fluid close to the walls. We
further discovered that most fetal hearts have a forward contraction wave,
where myocardial contraction and relaxation occurs in a wave from the inlet
region to the outlet region, and this was found to reduce energy needed for
ejection. References:
-
Wiputra H, Lai CQ, Lim GL, Heng JJW, Guo L, Soomar SM, Leo HL,
Biswas A, Mattar CNZ, Yap CH. "Fluid Mechanics of Human Fetal Right
Ventricles from Image-Based Computational Fluid dynamics Using 3D Clinical
Ultrasound Scans." Am J Physiol Heart and Circ Physiol. 2016 Dec
1;311(6):H1498-508. -
Wiputra H, Lim GL, Chu KC, R Nivetha, Soomar SM, Biswas A, Mattar
CNZ, Leo HL, Yap CH. "Peristaltic-Like Motion of the Human Fetal Right
Ventricle and its Effects on Fluid Dynamics and Energy Dynamics." Ann
Biomed Engr. 2017 Oct 1;45(10):2335-47. |
||||||||
|
|
|
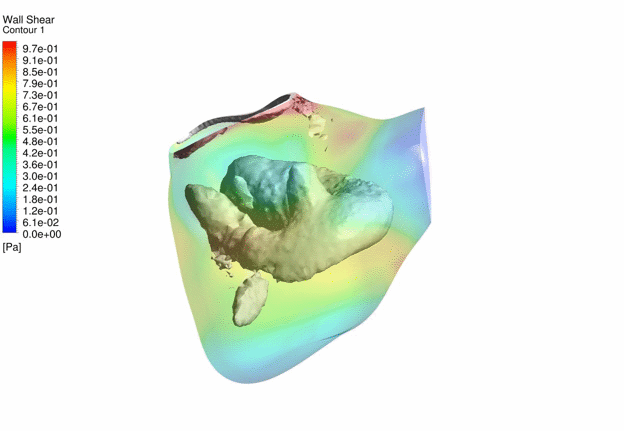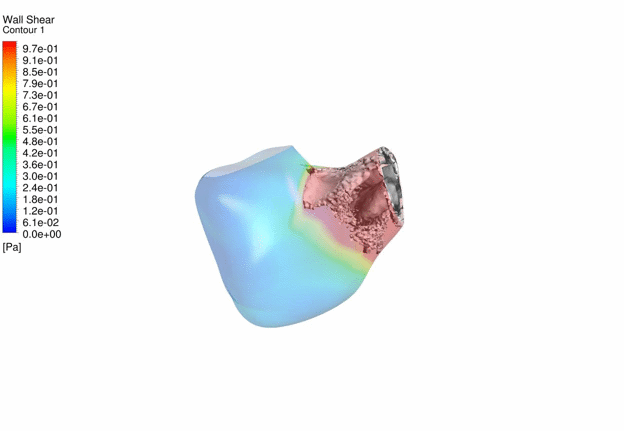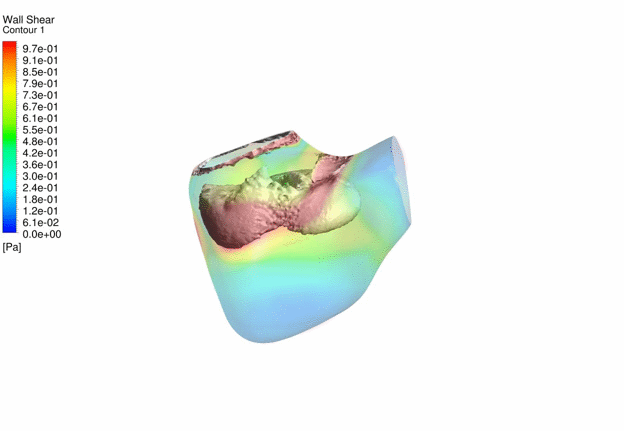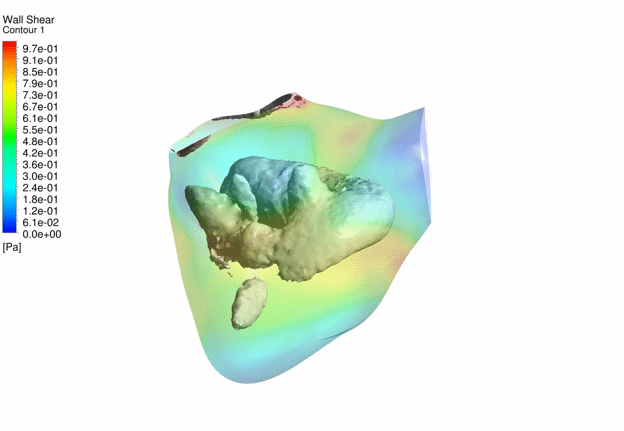
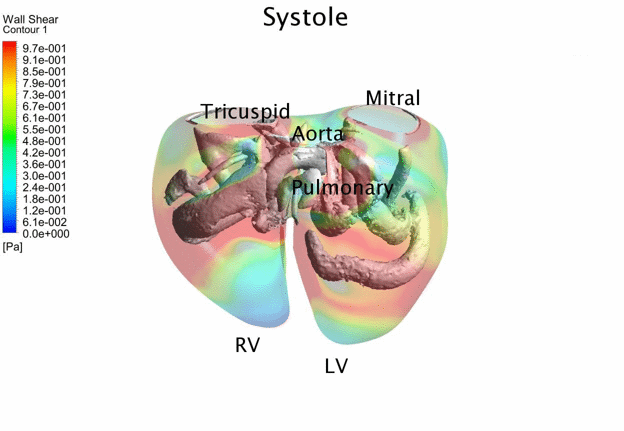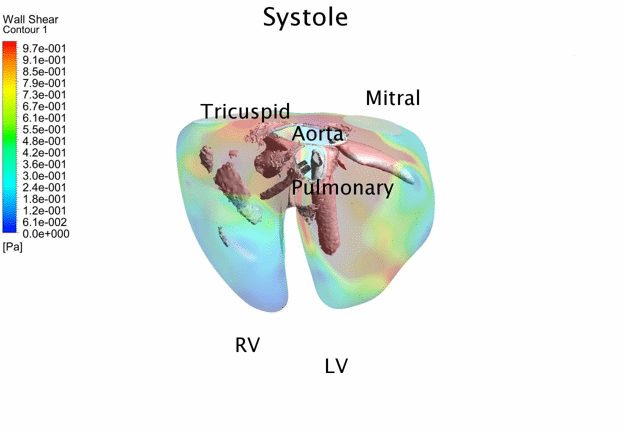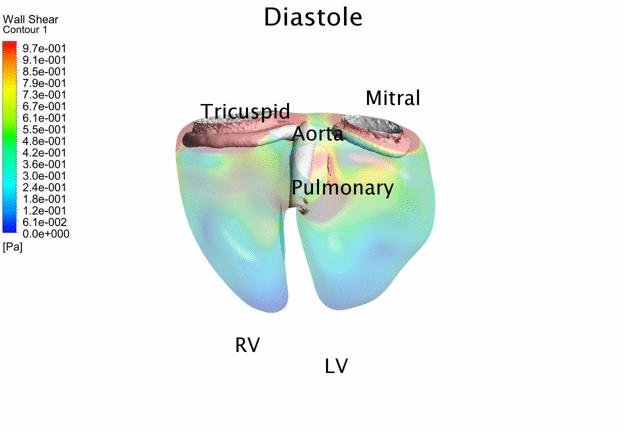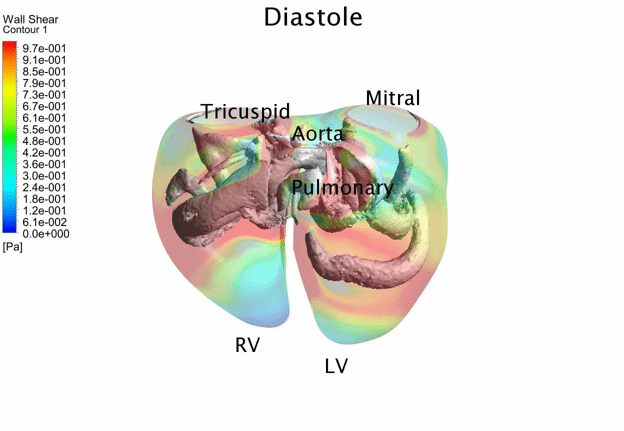
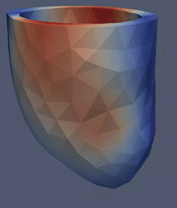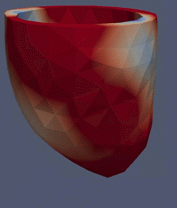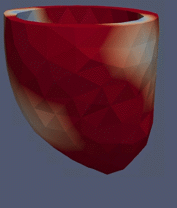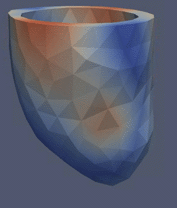
Computational Fluid
Dynamics Simulations of a 22 weeks old human fetal right ventricle based on
4D STIC clinical ultrasound images, demonstrating iso-vorticity surfaces and
wall shear stresses (as colour contours) |
|||||||
|
|
Fluid
Dynamics of Fetal Hearts with Tetralogy of Fallot We
applied our computational techniques on human fetal hearts with Tetralogy of
Fallot, to understand essential changes to fluid mechanics in this disease.
Among our discoveries were the presences of RV-to-LV diastolic shunting which
disrupts diastolic vortex rings in the LV, excessive flow rates and chaotic
flow patterns in the RV leading to high wall shear stresses compared to
normal hearts, elevation of pressures to the same extent in both ventricular
chambers above normal hearts, and higher work done necessary for ejection due
to outflow obstruction. Some TOF cases we investigated had prenatal RV
hypertrophy, and this might be related to elevated RV wall shear stresses,
since pressures in both chambers seemed to equilibrate via the septal defect. References:
- Wiputra H, Chen CK, Talbi E, Lim GL, Soomar SM, Biswas A, Mattar CNZ, Bark D, Leo HL, Yap CH. "Human Fetal Hearts with Tetralogy of Fallot have Altered Fluid Dynamics and Forces." Am J Physiol Heart and Circ Physiol. 2018 Sep 14;315(6):H1649-59. |
||||||||
|
|
|
Computational Fluid
Dynamics Simulations of a 32 weeks old human fetal heart with Tetralogy of
Fallot, based on 4D STIC clinical ultrasound images. Iso-vorticity surfaces
and wall shear stresses (as colour contours) are displayed. |
|||||||
|
|
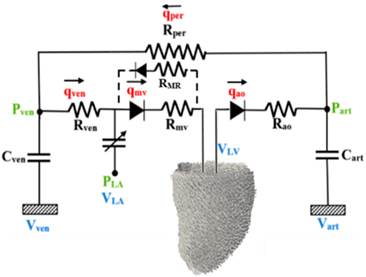
Finite
Element Simulation of Fetal Aortic Stenosis We
developed a fetal heart finite element model (FEM), based on reconstructions
from 4D clinical fetal echocardiography. This model modelled spatially
varying myofiber orientations, both passive hyperelastic tissue mechanical
properties and active tension, and a fetal Windkessel model. We validated
that our FEM model reflected the cardiac motion imaged from ultrasound scans
closely. We then investigated tweaked the model to reflect conditions during
fetal aortic stenosis and evolving HLHS. In this condition, fetal aortic
stenosis will cause high LV pressures, mitral regurgitation, drastically
reduced LV stroke volume. By birth, a majority of such cases will become
HLHS. We strive to understand the biomechanics of this disease.
Schematic of the
finite element model of the fetal left ventricle. Sample output from the FEM
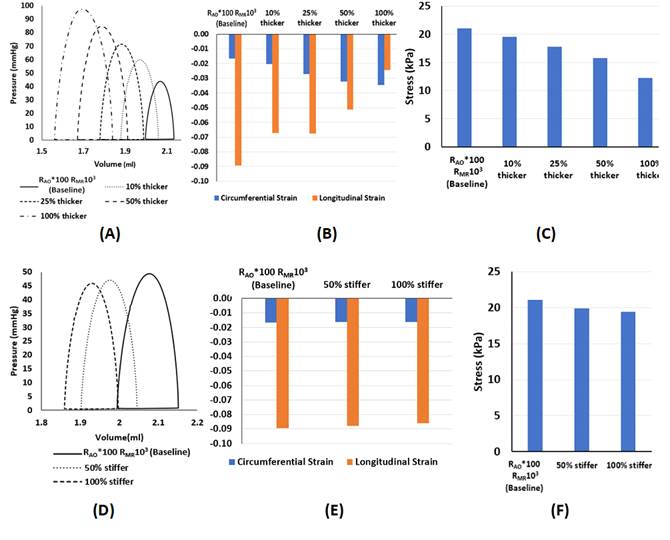
Effect of (A-C) LV
wall thickening, and (D-F) increasing LV stiffness, on the (A,D) PV loop,
(B,E) temporal-peak, spatial-averaged circumferential strain and longitudinal
strain, and (C,F) temporal-peak, spatial-averaged myocardial stress in the
fiber direction. From
our modelling results, Fetal aortic stenosis alone elevated pressures by
10-20 mmHg and could decimate stroke volume, and mitral regurgitation reduced
this elevation. Stenosis alone, however, did not lead to mitral regurgitation
velocities matching those measured clinically, and only when myocardial wall
hypertrophy is modelled could we achieve this match. Typical extent of LV
hypertrophy, however, resulted in excessive LV pressures well above clinical
measurements, thus suggesting that reduction of myocardial contractility also
accompanied the disease. Increasing the passive stiffness of the LV, however,
did very little to affect heart function, suggesting that fibroelastosis is a
by-product of the disease, and does not impede heart function. References:
-
Ong CW, Ren M, Wiputra H, Mojumder J, Chan WX, Tulzer A,
Tulzer G, Buist ML, Mattar CNZ, Lee LC, Yap CH. "Biomechanics
of Human Fetal Hearts with Critical Aortic Stenosis." Ann
Biomed Eng. 2020 Nov 11:1-6 |
||||||||
|
|
Future
Work We
are currently studying the biomechanics of fetal aortic stenosis to
understand why it has a high likelihood of causing HLHS at birth. We are also
investigating fetal aortic balloon valvuloplasty and other fetal heart
interventions, to understand their effects on the fetal heart function,
biomechanics, and development, so as to help improve these interventions. |
||||||||