|
Cardiovascular Biomechanics and A.I. Laboratory |
|
|
Imperial College London, Department of bioengineering |
|
Zebrafish
Embryonic Heart Biomechanics |
|||||||
|
The
zebrafish embryo is a well-studied animal model of embryonic heart
development. We performed a careful characterization of the zebrafish
embryonic hear biomechanics to understand it better. A similar approach to
our other fetal and embryonic studies was adopted. We employed various
high-resolution microscopy to scan the embryonic heart in 4D, apply our
validated cardiac motion estimation algorithm to extract cardiac movements,
and then perform both computational fluid dynamics to understand cardiac fluid
mechanics, and finite element modelling to understand myocardial mechanics,
to understand the embryonic heart function and support mechanobiology
investigations. Fluid
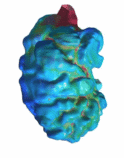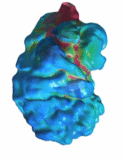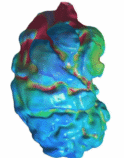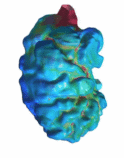
Mechanics of the Embryonic Heart Trabeculation We employed a high-resolution spinning disc
confocal microscopy of zebrafish line with endothelial membrane fluorescence
for our CFD flow simulations. This allows the accurate location the
blood-endocardium boundary for the simulations, which we found was important,
using myocardial fluorescence appears to underestimate walls shear stresses.
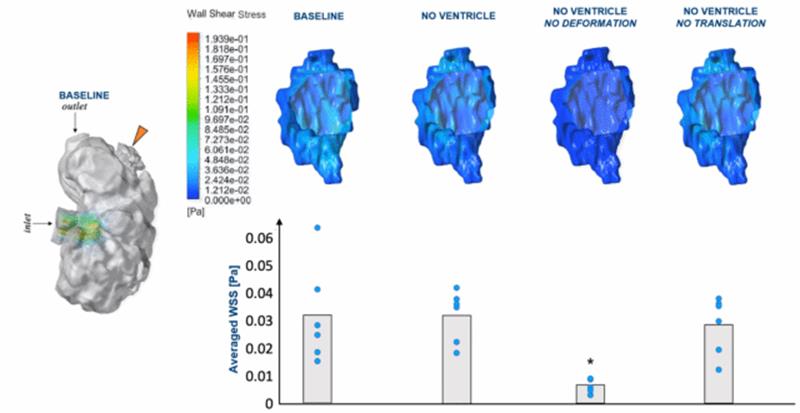
Our simulations showed that trabeculations
enhanced wall shear stresses at the ridges and reduced wall shear stresses at
the grooves. We studied individual inter-trabeculation fluid spaces, and
found that the contraction motion squeezing on these spaces is the main
driver of wall shear stresses on endothelium within these spaces, rather than
flow induced by fluid in the main chamber, or translation of the chamber.
Further we observed endothelial-hematopoetic cells within the
inter-trabecular spaces that enhanced wall shear stresses.
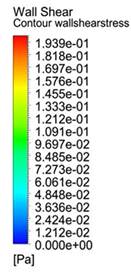
What is the main driver of fluid forces in the
inter-trabecular space?
Simulations of individual trabeculation spaces under various scenarios,
Baseline - simulated together with the rest of the ventricle; No Ventricle -
detached from ventricle during simulation; No Deformation - detached from
ventricle with no deformational motion; No Translation - detached from
ventricle with no translation motion. Results show that removing the
ventricle or translation motion did not affect wall shear stresses much, but
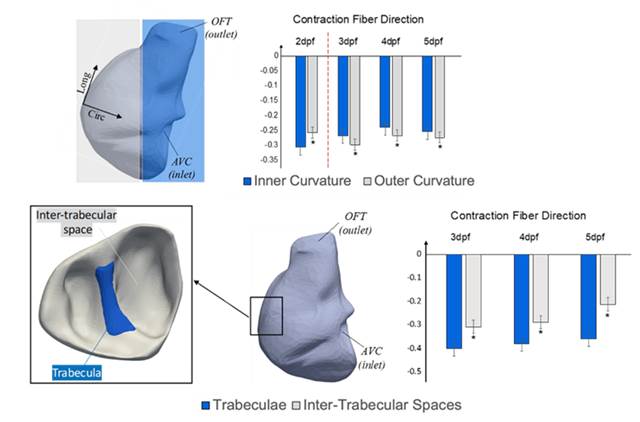
removing deformational motion drastically reduced it. Interestingly, we observed that there was not much
difference in the wall shear stress magnitude or oscillatory index at regions
of the embryonic heart that trabeculated (outer curvature region), versus
regions that did not trabeculate (Inner curvature region). This suggested
that fluid forces stimuli alone may not be enough to induce trabeculation
formation.
Reference: - Cairelli AG, Chow RW, Vermot J, Yap CH. "Fluid Mechanics of the Zebrafish Embryonic Heart Trabeculation." PloS Comput Biol. 2022 Jun 6;18(6):e1010142 Tissue
Mechanics of the Embryonic Heart Trabeculation To
understand the function and mechanobiological origins of embryonic heart
trabeculations, we performed image-based finite element modelling. We find
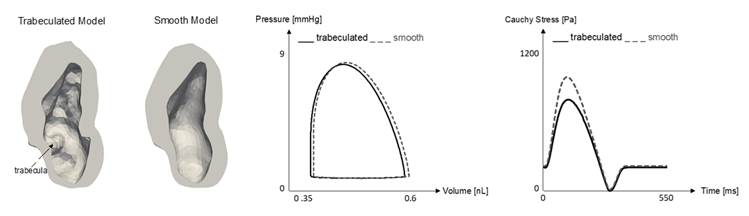
that trabeculations enhances deformability of the myocardium, allowing the heart to undergo higher strains
where there are trabeculations, and trabeculations themselves undergo higher
strains than surrounding myocardium. Further, artificially smoothed hearts
(removing trabeculation) with the same myocardial mass could sustain the same
cardiac function with reduced myocardial tensile stresses. These suggest that
trabeculations have the function of enhancing deformability and reducing
stresses.
Our workflow of image-based finite element modelling of zebrafish
embryonic heart
Image-tracking quantification of myocardial strains showed that
regions with trabeculation has higher strain, while the trabeculations
themselves showed higher strains than surrounding myocardium. This suggests
that trabeculations play a role to enhance tissue deformability.
Finite Element Simulations showed that if trabeculations are removed
(smooth model), stresses are higher, suggesting that trabeculations play a
role of reducing myocardial stresses. Imaged-Based
CFD Simulation of Zebrafish Embryonic Heart In
our previous simulations, we characterized the general wall shear stresses
characteristics of the normal zebrafish embryonic heart, which were found to
be elevated at the atrioventricular inlet and near the outlet. Wall shear
stresses were biphasic, corresponding to inflow and outflow, and consequent
to the highly viscous, low Reynolds Number environment, wall shear stresses
were elevated near the inner curvature of the embryonic heart, and lower at
the outer curvature. The heart was found to have a wave-like contraction
motion, where the inlet would contract earlier than the outlet. This was
found to favour ejection, as it saves energy required for ejection. Reference: - Foo YY, Pant S, Tay S, Imangali N, Chen NG, Winkler C, Yap CH. "4D modelling of fluid mechanics 1 in the zebrafish embryonic heart." Biomech Model Mechanobiol. 2020 Feb;19(1):221-32.
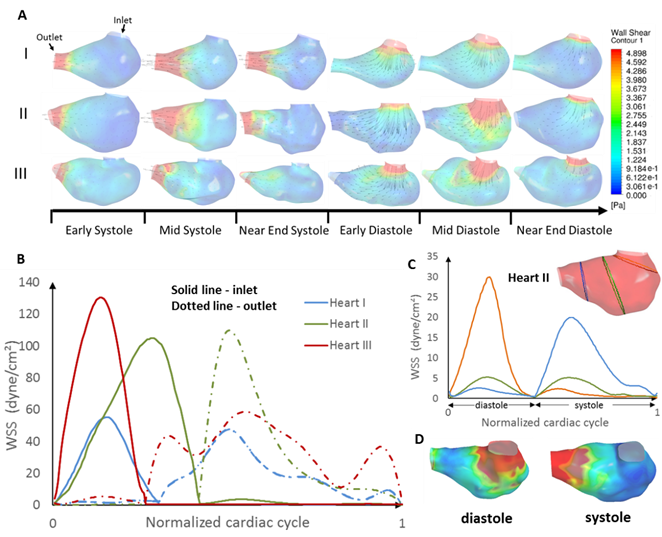
(A)
Contour maps of wall shear stress and velocity vectors along the longitudinal
cross-section in three normal ventricles over the cardiac cycle. (B) WSS
averaged across the cross-section, plotted against time for the three hearts.
Solid line and dash line represent the measurements at the near-inlet region
and near-outlet region respectively. The first hump in the waveform
correspond to diastole. (C) WSS averaged across the cross-section, plotted
against time, at three different regions for heart II over the course of the
cardiac cycle. (D) WSS of heart II at peak diastole and peak systole
respectively in 3D view, demonstrating that the inner curvature of the
ventricle experienced higher WSS. |