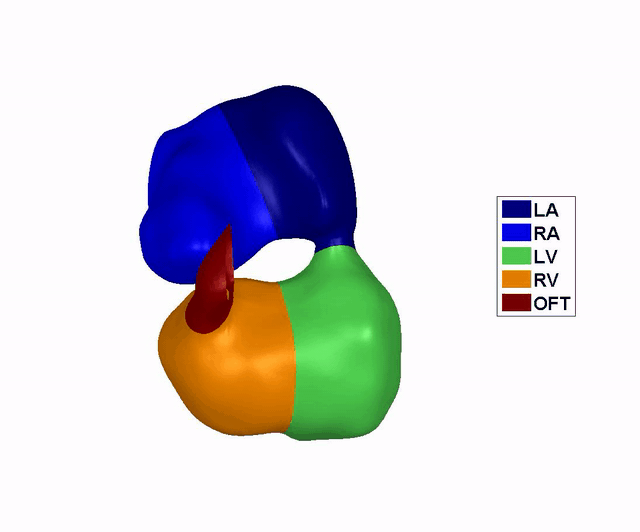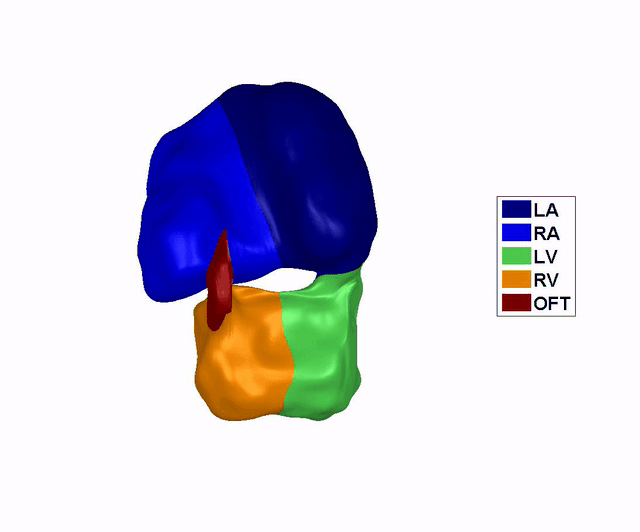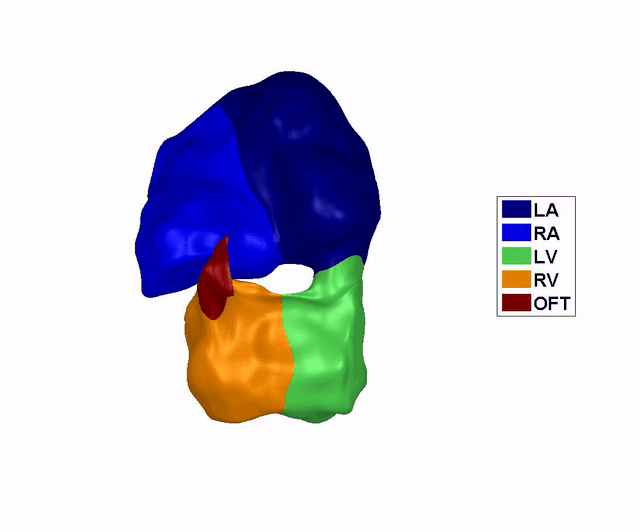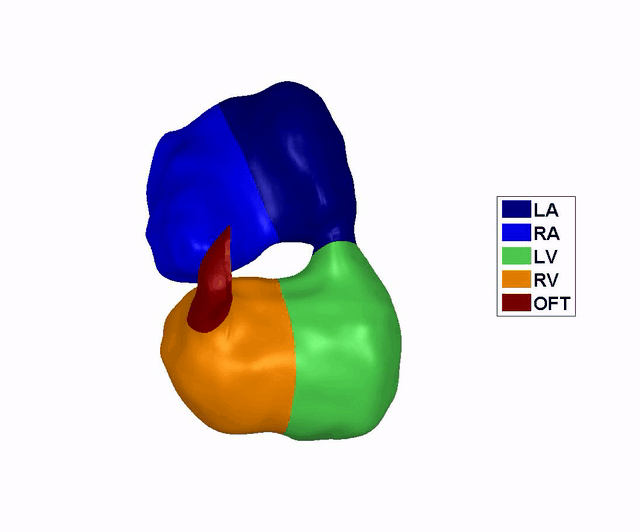|
Cardiovascular Biomechanics and A.I. Laboratory |
|
|
Imperial College London, Department of bioengineering |
|
Embryonic
Heart Biomechanics |
||||||||||
|
The
embryonic heart is the first organ to develop. It undergoes a fascinating
developmental process, starting out as a simple tube and develops into a
4-chamber structure by week 8 of gestation. We hypothesize that mechanical
forces are important stimuli to proper early cardiac development, seek to
understand the biomechanics of the embryonic heart via novel imaging, image
processing, and biomechanics simulations. Our
approach was to employ high frequency ultrasound imaging and processing,
apply image registration and other mathematical modelling to extract and
define cardiac movements, and then perform dynamic mesh computational fluid
dynamics will be performed to understand embryonic heart flow patterns and
forces. 4D
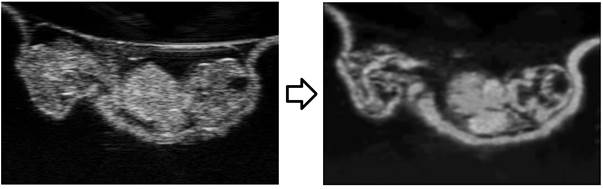
Ultrasound Imaging of Small Animal Embryos In
ultrasound images of small animal embryos, there is a lack of contrast
between blood and tissue spaces, however, blood spaces has dynamic speckles
while tissue spaces has persistent ones. We thus developed a technique to use
ensemble averaging using quadratic means to differential between tissue and
blood spaces. This way, we could reconstruct the 4D dynamic motions of the
embryonic heart, and demonstrated this in the chick embryo. We are applying
the same technique in rat embryos and will share this soon.
(Left) raw
ultrasound images of a 4.5 days chick embryo. (right) the same
image after image processing to distinguish blood and tissue pixels.
Reconstruction of
the Dynamic Motion of the (left) 4.5 days and the (right) 5.5 days chick
embryonic heart, obtained using phase averaging of ultrasound images, and
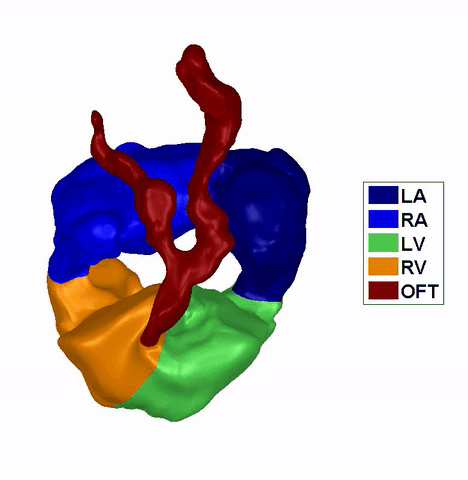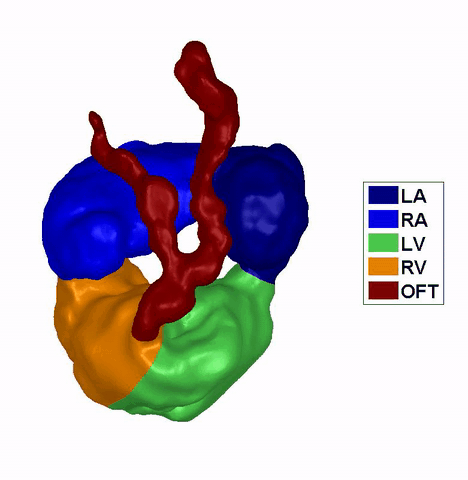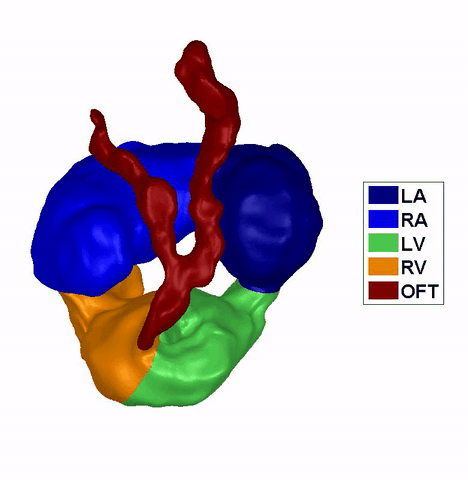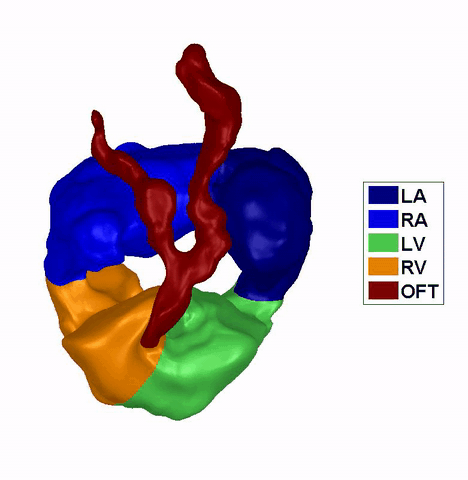
spatial and temporal correlation. LA: left atria, RA: right atria, LV: left
ventricle, RV: right ventricle, OFT: outflow tract. References: -
Tan GXY, Jamil M, Tee NGZ, Zhong L, Yap CH. "3D Reconstruction
of Chick Embryo Vascular Geometry Using Non-Invasive High-Frequency
Ultrasound for Computational Fluid Dynamics." Ann Biomed Engr.
2015; 43(11): 2780-2793. -
Ho S, Tan GXY, Foo TJ, Phan-Thien N, Yap CH. "Organ Dynamics
and Fluid Dynamics of the HH25 Chick Embryonic Cardiac Ventricle as Revealed
by a Novel 4D High-Frequency Ultrasound Imaging Technique and Computational
Flow Simulations." Ann Biomed Engr.† 2017 Oct 1;45(10):2309-23. Computational
Fluid Dynamics of Chick Embryonic Ventricle and Outflow Tract Using
this imaging technique, we performed dynamic mesh CFD flow simulations, and
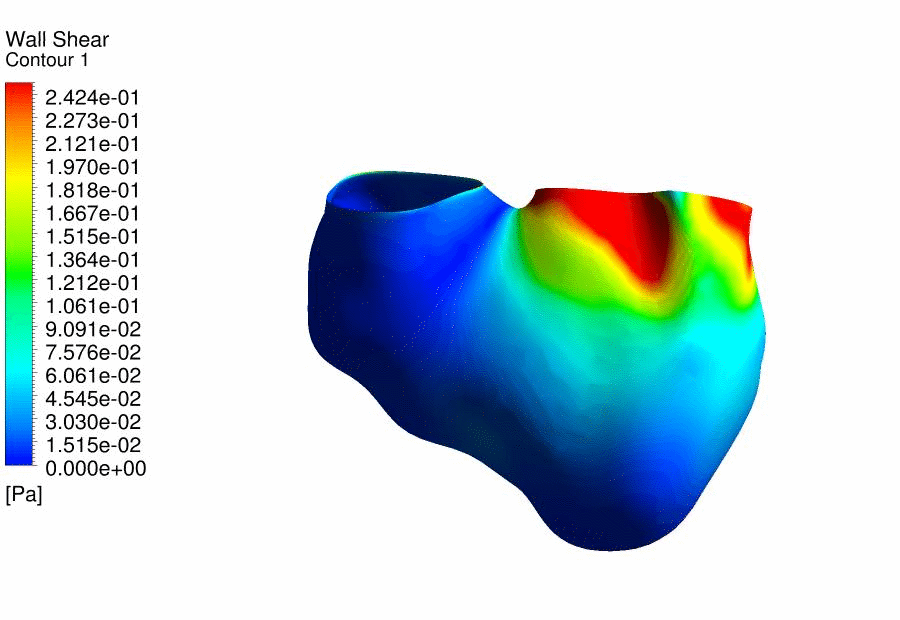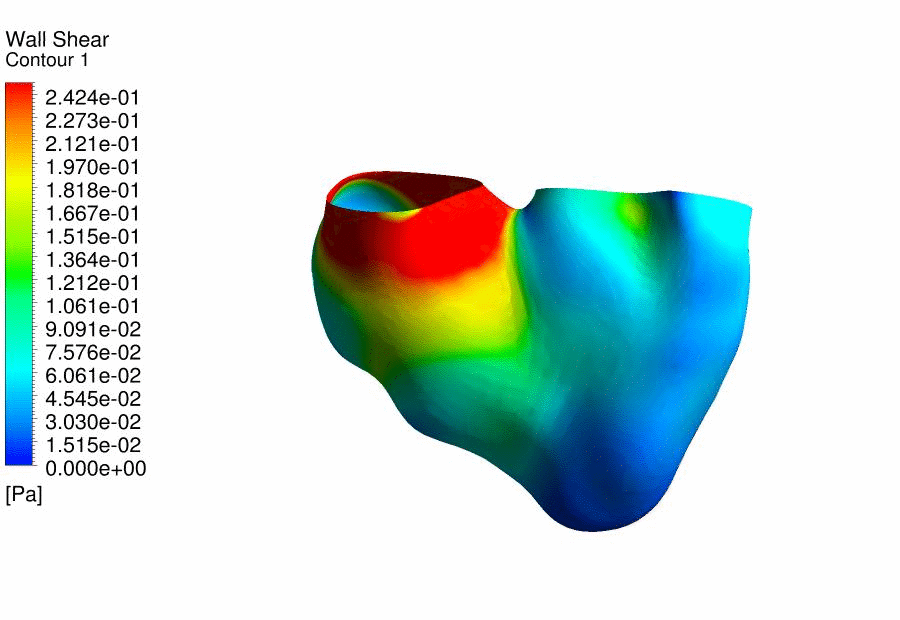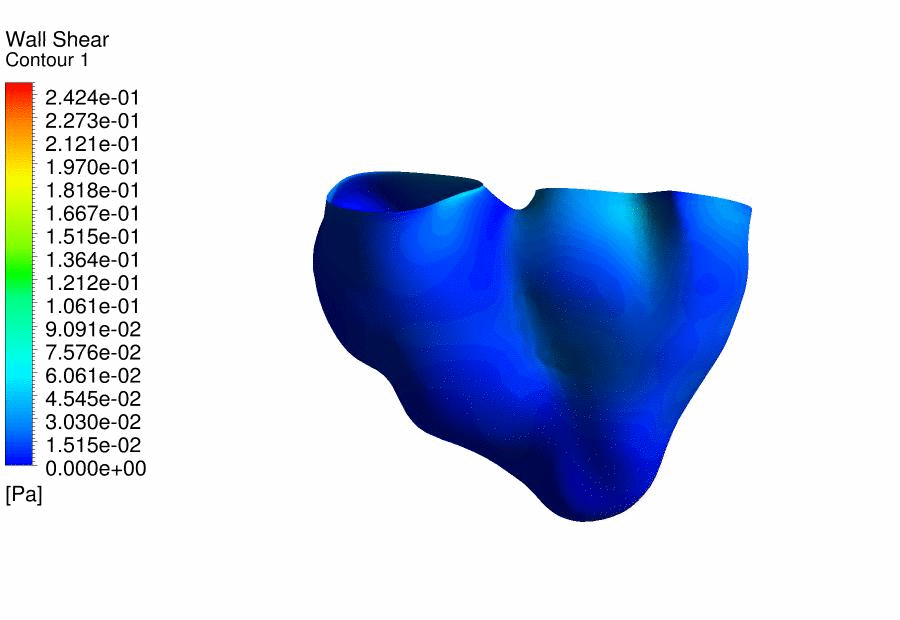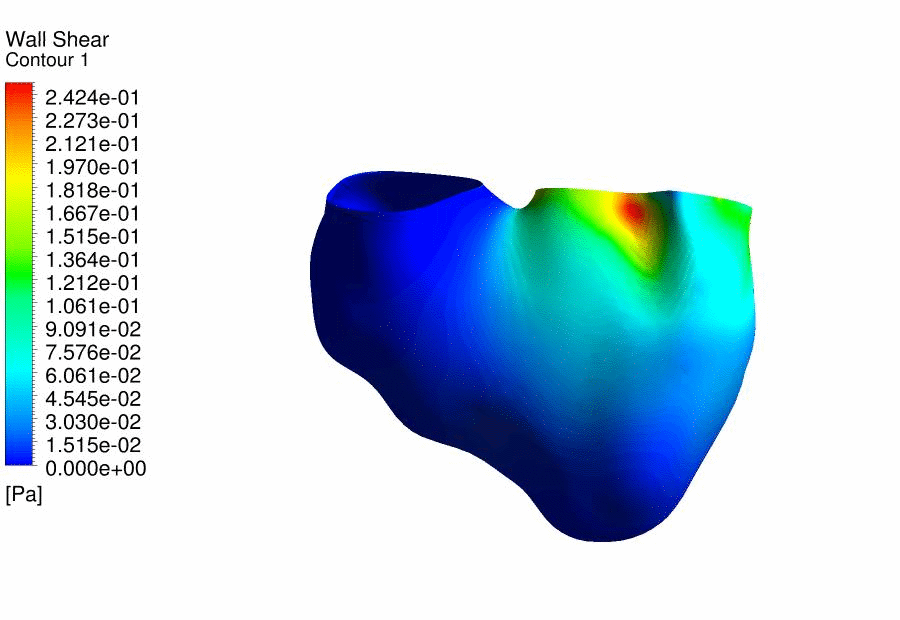
discovered that there were magnitude differences in the wall shear stresses
between the left side and right side of the embryonic ventricle, which could
play a role into the different morphology of the RV and LV walls. |
|||||||||||
|
|
|
Results
of CFD Flow Simulations of a 4.5 days old chick embryonic heart,
demonstrating the wall shear stresses. |
|||||||||
|
|
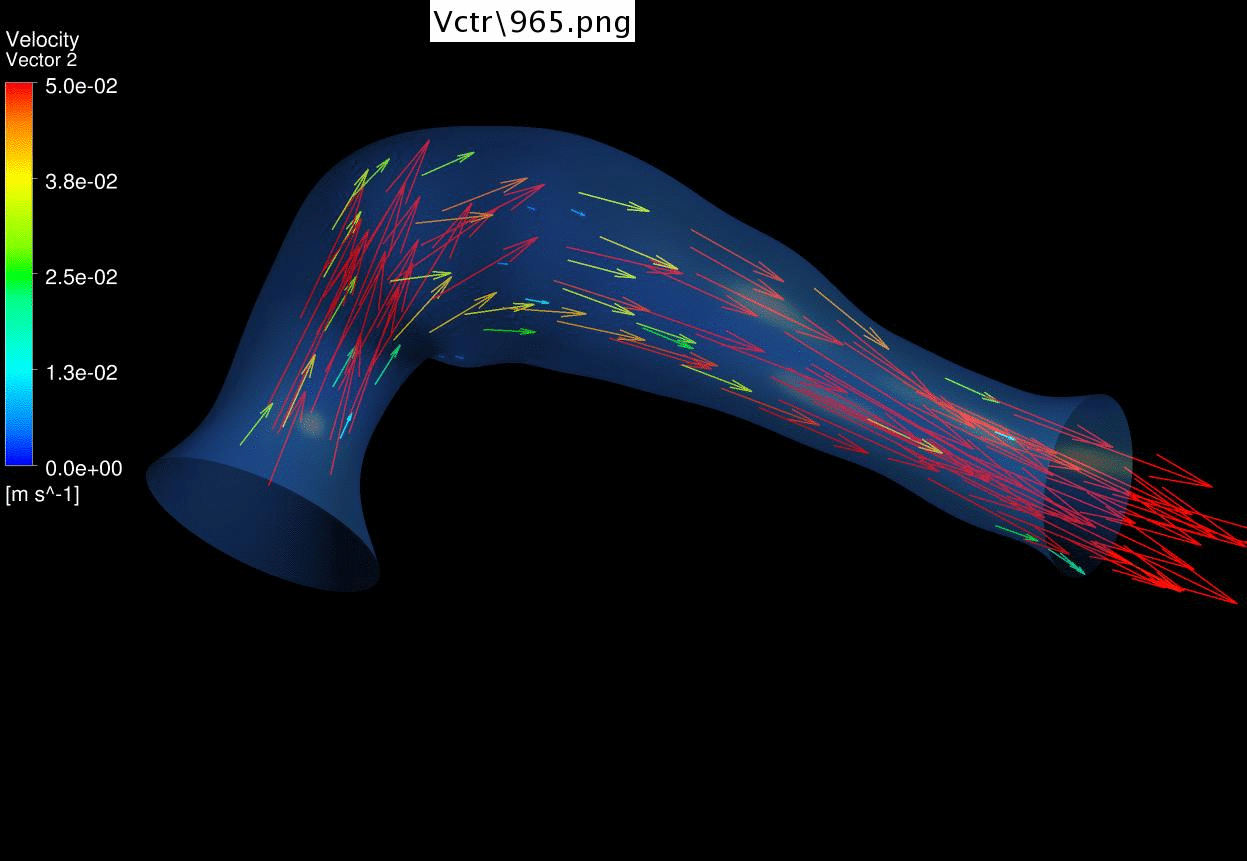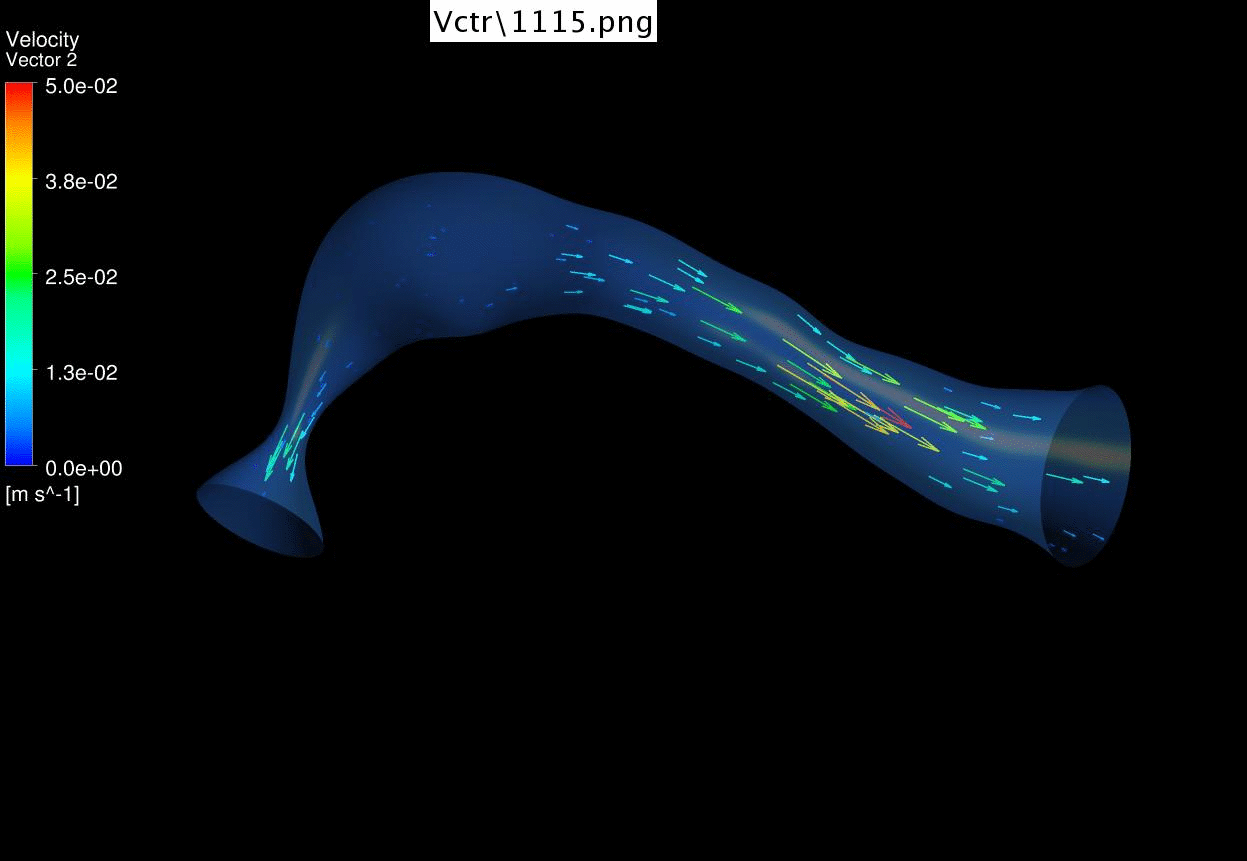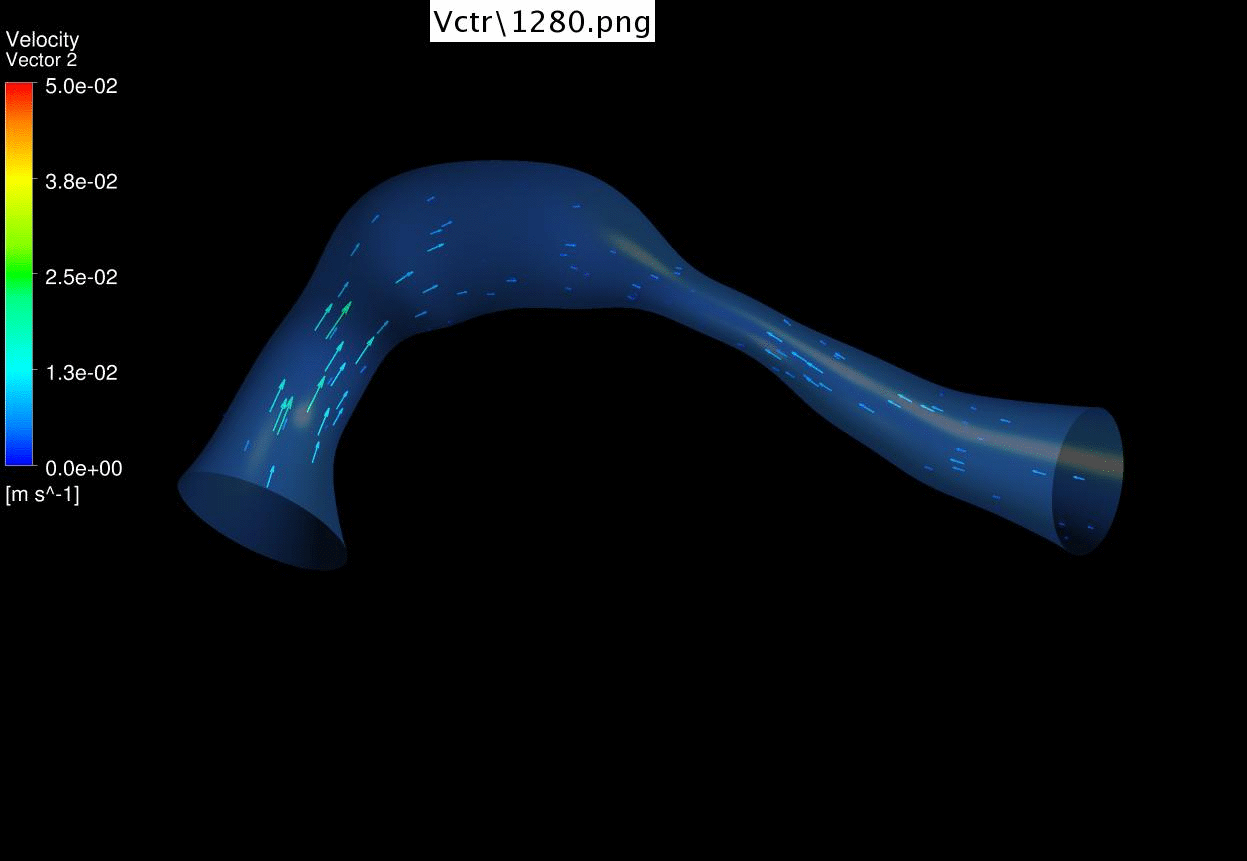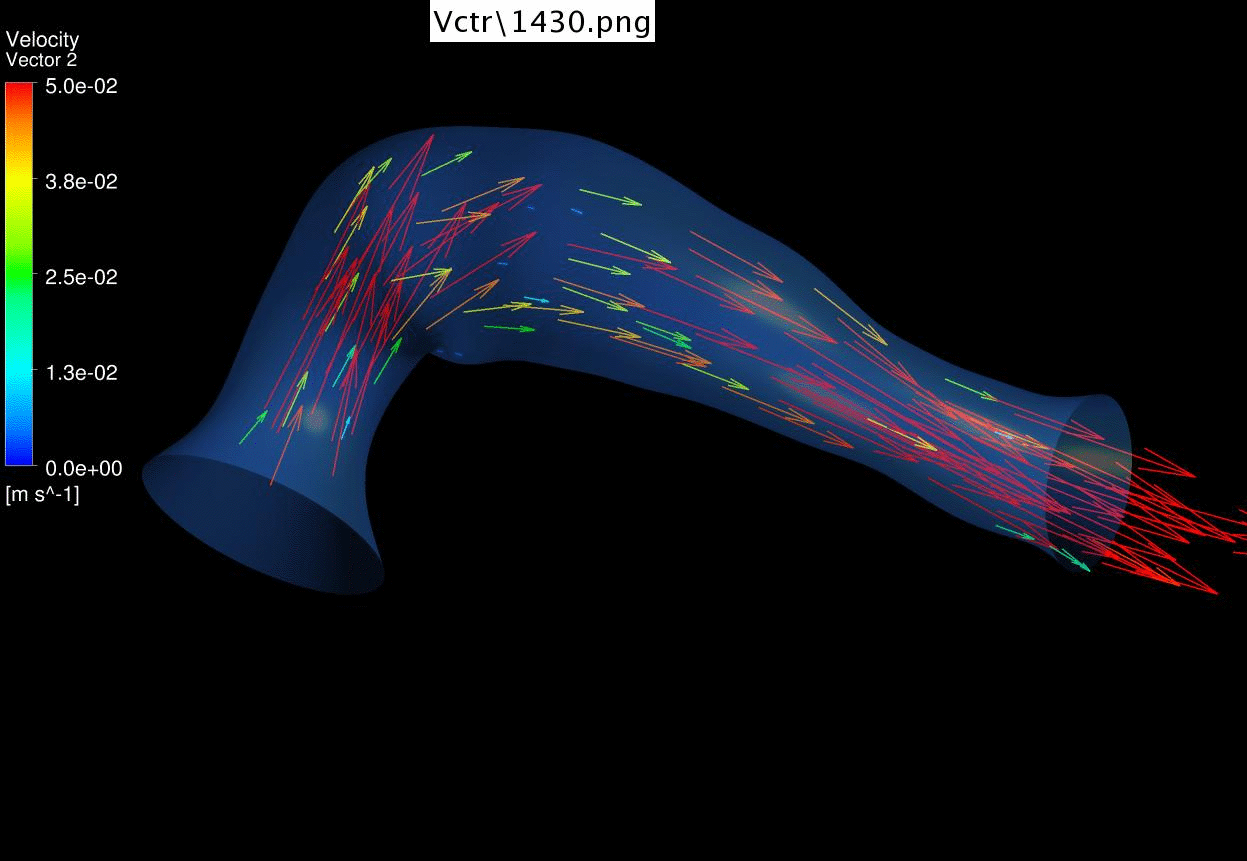
We
further performed CFD of the outflow tract at 4.5 days gestation, and found
that the there’s excessive oscillatory wall shear stresses at the outflow
tract cushions, which could be important stimuli for their eventual
development into heart valves and aortico-pulmonary septum. Further, we
discovered that there’s a double helical flow structure during peak flow
phases, which have similarities to the geometry of the aortico-pulmonary
septum, suggesting the possibility that flow is guiding the septation. This
work is currently being published. |
||||||||||
|
|
|
Results of CFD Flow
Simulations of a 4.5 days old chick embryonic cardiac outflow tract,
demonstrating flow velocities vectors and magnitude. |
|||||||||
|
|
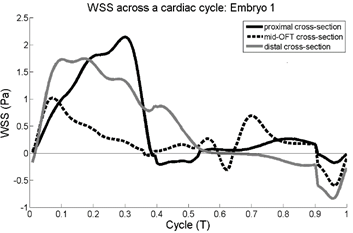
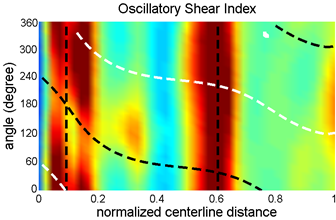
(left) Plots of Streamwise wall shear stress versus cardiac
cycle time. (right) Oscillatory index at various locations of the outflow
tract walls. Vertical dotted lines are the location of outflow tract
cushions, while and black curved dotted lines represent the outer and inner
curvatures.
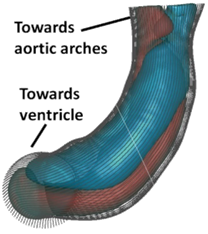
At the time point just before septation (4.5 days gestation),
the outflow tract featured a two columns of helical flow structures, and the
dividing line between the two structures seem to have geometric similarities
to the eventual aortico-pulmonary septation, suggesting a possibility that
flow is guiding septation. Reference: -
Ho S, Tan GXY, Foo TJ, Phan-Thien N, Yap CH. "Organ Dynamics
and Fluid Dynamics of the HH25 Chick Embryonic Cardiac Ventricle as Revealed
by a Novel 4D High-Frequency Ultrasound Imaging Technique and Computational
Flow Simulations." Ann Biomed Engr.† 2017 Oct 1;45(10):2309-23. Embryonic
Heart Atrio-Ventricular Fluid Dynamics Using
a novel image registration technique, we extracted the wall motions of both
the atria and ventricle of the embryonic heart, and performed computational
simulations on it. One interesting finding we had was that the embryonic
atrial appendages appeared to be playing the role of enhancing atrial
function. They are the most contractile part of the atria, and accounts for
32% of atrial stroke volume and 42% of atrial work done, although they only
occupy 20% of the atrial volume. Proper atrial function is likely important
for providing the right pattern of wall shear stresses to stimulate the heart
towards normal growth and remodelling. -
Ho S, Chan WX, Nhan PT, Yap CH. "Organ Dynamics and Hemodynamics of the Whole HH25
Avian Embryonic Heart, Revealed
by Ultrsound Biomicroscopy, Bounday Tracking, and Flow
Simulations." Scientific Reports. 2019 Dec 2;9(1):1-4. |
||||||||||
|
|
|
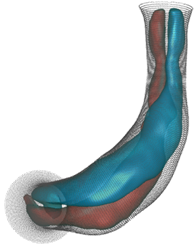
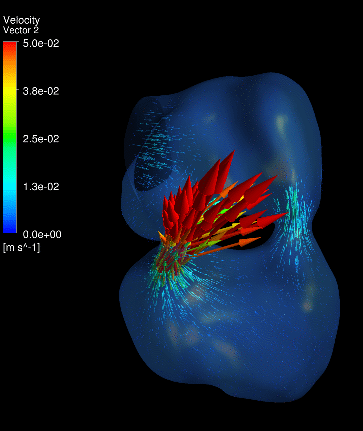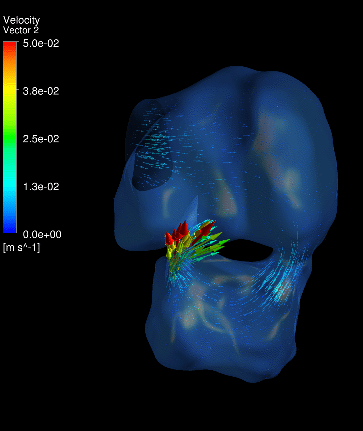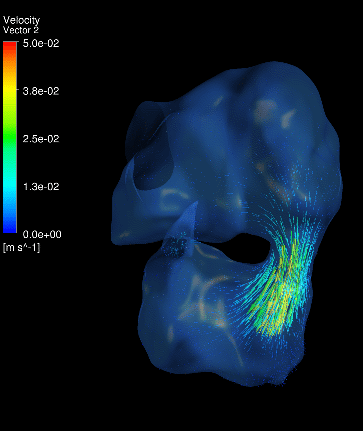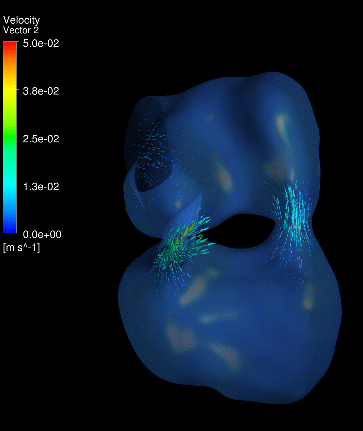
Dynamic mesh CFD
simulations of flow in the normal (control) 4.5 days old chick embryonic
atria and ventricle |
|||||||||
|
|
Atrial appendages (circle) are among the most contractile
structures in the embryonic heart, and appear to have the function of
enhancing atrial pumping. We further performed similar studies
on embryos after left atrial ligation at E3.5, which is an animal model of
hypoplastic left heart syndrome (HLHS morphology observed by E6.5). Here, we
discovered that the LV started to become smaller while the RV started to
enlarge in compensation at E5.5, even before ventricular septation is
complete. Closer investigation showed that LAL caused the shape changes to
the heart, moving the atrioventricular junction medially, and causing the
ventricular apex to become sharper. These caused changes to ventricular flow
pattern, and created weak and oscillatory flow near to the LV free wall,
which we believe is related to why the smaller LV developed. |
||||||||||
|
|
|
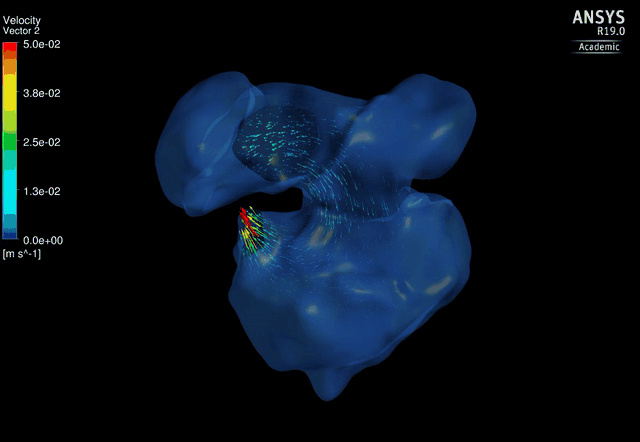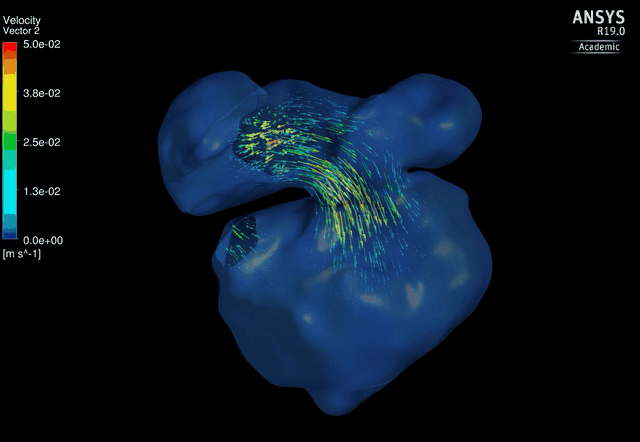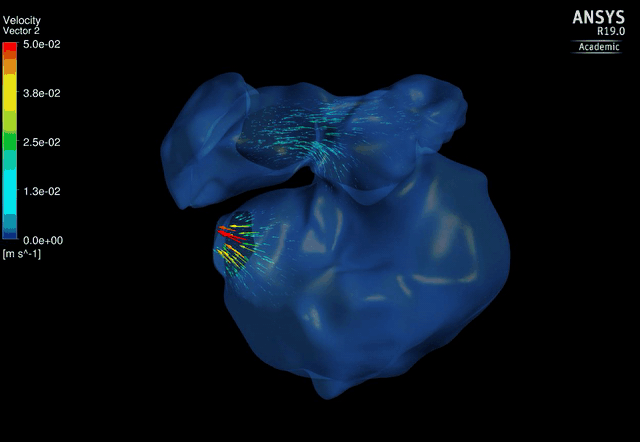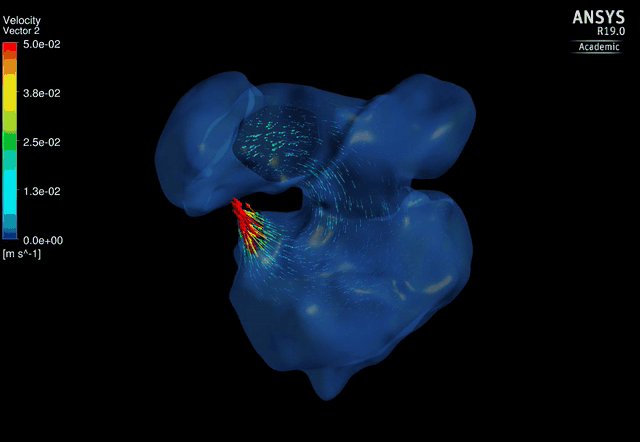
Dynamic mesh CFD
simulations of flow in the 4.5 days old chick embryonic atria and ventricle,
after Left-Atria-Ligation surgery to induce Hypoplastic Left Heart Syndrome. |
|||||||||
|
|
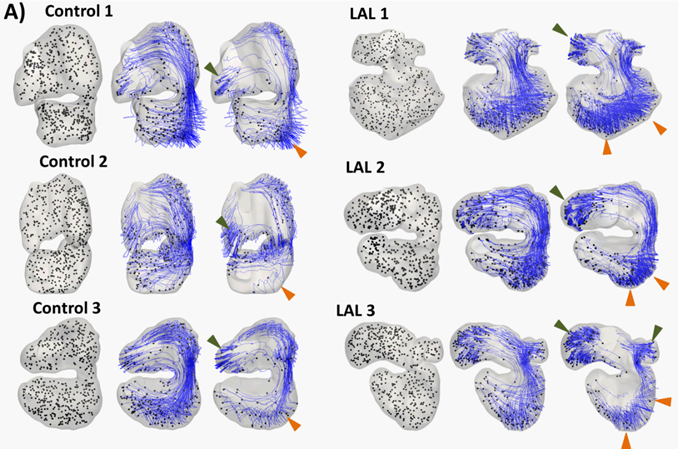
The pathlines of particles in the embryonic heart after 0, 2,
and 5 cardiac cycles for HH25 hearts (both control and left atrial ligated).
Green arrow demarcates region of high particle retention in the atrial
appendage. Orange arrow demarcates region of high particle retention in
ventricle apex and left ventricular free wall. |
||||||||||
|
|
Future
Work We are
currently investigating the mechanobiological expressions of the chick embryonic
atrial ligation model of HLHS, and attempting to find the exact pathways by
which such abnormal flow conditions can lead to the hypoplastic left heart in
this animal model. We are also attempting to develop finite element model of
the chick embryonic heart, so that we can model the growth and remodeling in
the disease model. |
||||||||||